ARC Medical’s Novel Fluid Medical Device JOCOAT for Surgical Adhesions Deployed in Surgical Patients for the First Time
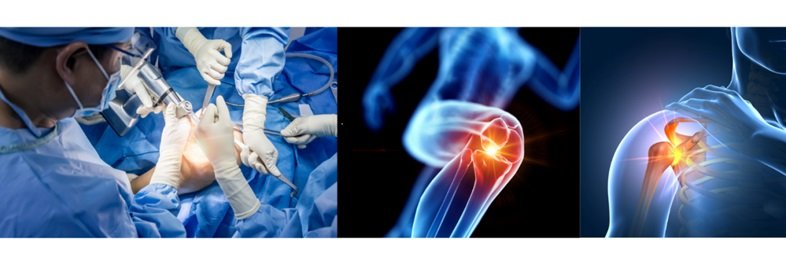
Vancouver, British Columbia, Canada (Feb 9, 2024) – ARC Medical Inc. (ARC), a leading innovator in advanced medical devices, today announced the first deployment of its novel JOCOAT™ fluid medical device for the prevention or reduction of surgical adhesions in two surgical patients. One patient underwent knee surgery and the other patient had shoulder surgery. Both surgeries took place in December 2023 in Canada.
The surgeries and applications of the device were performed by arthroscopy (a type of minimally invasive surgery). At the end of surgery and immediately prior to closure, 10 mL of ARC’s JOCOAT™ fluid medical device were injected into the afflicted joint of each patient. The application of all of ARC’s fluid medical devices into the surgical area aims to prevent or reduce the formation or reformation of surgical adhesions, which are abnormal fibrous growths between tissues that can negatively impact patient recovery including mobility and long-term health.
“The use of ARC’s fluid medical devices in surgical patients follows the completion of ARC’s Phase 1 safety clinical trial and a comprehensive battery of preclinical ISO 10993 and other biocompatibility, safety and efficacy studies,” stated Dr. Chris Springate, CEO of ARC.
“The safety and efficacy of our fluid medical devices are being investigated through clinical trials.”
– Dr. Chris Springate, CEO of ARC“ARC is developing IPCOAT™ fluid for the prevention or reduction of surgical adhesions in gynecological and abdominal surgeries and we are developing JOCOAT™ fluid for orthopedic surgeries, including knee, shoulder, ankle and hip joints,” added Dr. Springate.
“The knee surgery patient had an earlier knee surgery from which she struggled to regain her motion due to intra-articular adhesions that formed after the initial surgery. Prior to knee revision surgery in December 2023, the patient had only 95 degrees of range of motion and could not spin (ride a stationary bike),” stated Dr. Robert Litchfield, ARC’s Chief Medical Officer. “During knee revision surgery, the surgeon removed all intra-articular adhesions and then applied ARC’s JOCOAT™ fluid medical device. At approximately six weeks after knee revision surgery the patient’s range of motion had increased 40 degrees to 135 degrees and she is spinning regularly,” added Dr. Litchfield.
“The shoulder surgery patient had an earlier shoulder stabilization procedure for dislocations and following that procedure developed early osteoarthritis and a tightening of the shoulder capsule from which he suffered significant motion loss. Prior to shoulder revision surgery in December 2023, the patient had only 90 degrees of range of motion in his shoulder, lifting his arm no higher than straight out to the side,” said Dr. Litchfield. He added, “During shoulder revision surgery, the surgeon removed bone spurs, released the tightened capsule, and then applied ARC’s JOCOAT™ fluid medical device.”
“At approximately six weeks after shoulder revision surgery the patient’s range of motion had increased 60 degrees to 150 degrees and he is able to lift his arm almost all the way over his head”
– Dr. Robert Litchfield, ARC’s Chief Medical Officer
Dr. Litchfield explained, “Surgical adhesions often reform thicker and stronger with poor mobility and function following revision surgery. Given that there are no products on the market to prevent adhesions following orthopedic surgery, these two surgical revision patients were ideal candidates for ARC’s JOCOAT™ fluid medical device. We are particularly pleased with these patients’ improvements in range of motion and function. Medical staff will continue to closely monitor the patients’ recoveries and collect additional safety and efficacy data during routine surgical follow-ups. These data are instrumental for informing the design of clinical trials as ARC seeks regulatory approvals.”
The earlier completed Phase 1 clinical trial was a randomized, placebo controlled, double-blinded, first-in-human clinical trial carried out with 76 healthy volunteers. Each participant was injected into the abdominopelvic cavity under ultrasound guidance with ARC’s gynecological/abdominal IPCOAT™ fluid medical device or placebo control Lactated Ringer’s Injection USP (LRS). The Phase 1 clinical trial comprised two parts. In Part 1, the primary objective was to evaluate the safety and tolerability of a single ascending volume of ARC’s IPCOAT™ compared with placebo control LRS. In Part 2, the primary objective was to evaluate if there was any potential interaction between ARC’s IPCOAT™ and the commonly used anticoagulant for venous thromboembolism prophylaxis, enoxaparin sodium (Lovenox®). The results from the Phase 1 clinical trial showed that ARC’s IPCOAT™ fluid medical device was well tolerated and accepted at up to and including 3 mL IPCOAT™ / kg body weight without or with the concomitant use of enoxaparin sodium.
About surgical adhesions
Surgical adhesions are the most common surgical complication and preventing or reducing surgical adhesions is a large unmet medical need. Surgical adhesions are abnormal fibrous growths that occur between tissues and organs following surgical procedures, even in cases with meticulous, minimally invasive surgical technique. Surgical adhesions commonly occur in gynecological, abdominal, knee, shoulder, hip and many other surgeries. Patients who form surgical adhesions, and depending on the type of surgery, are at risk of chronic pain, restricted mobility, organ dysfunction, infertility, bowel obstruction and sometimes death. Once surgical adhesions form they become permanent and are removed only with a second, expensive surgery or procedure to cut apart or break the adhesions; and in these patients the adhesions often reform thicker and stronger than before.
Current methods of reducing adhesions have limitations in application and effectiveness. For gynecological and abdominal surgeries, surgical adhesion reduction strategies may involve the use of biodegradable films or viscous gels that can be difficult to handle and challenging or not approved for application in laparoscopic procedures (a type of minimally invasive surgery); and are only effective at the location where they are applied (surgeons cannot accurately predict where all surgical adhesions might form) – and the other option is a volume of liquid that does not form a physical barrier film and instead is thought to work through hydroflotation; that is, to float organs to keep them apart. For orthopedic surgeries, no products are approved in North America nor in many other territories for the reduction of surgical adhesions.
Additional device information
ARC’s fluid medical devices are undergoing investigation for safety and effectiveness, which have not been established by any regulatory agency, including Health Canada and the US FDA. ARC’s medical devices have not been granted a market authorization or license by any regulatory agency, including Health Canada and the US FDA; and are not otherwise available in the United States.
ARC’s fluid medical devices comprise an aqueous, fluid solution of a patented, high molecular weight polymer that is extracted from seaweed and then chemically modified and ultrapurified in ARC’s ISO 13485 certified and cGMP-compliant manufacturing facility in Canada. The final devices are manufactured and sterilized by an FDA inspected, GMP-compliant contract manufacturing organization in the US.
ARC’s fluid medical devices are regulated by Health Canada as medical devices because the devices’ mode of action is physical and mechanical; and the devices are not known to have any chemical or pharmacological action or be dependent on degradation, in the prevention or reduction of surgical adhesions. Preclinical radiolabel and other studies demonstrated that following application of ARC’s fluid medical devices into the surgical area, the devices flow and form a temporary physical barrier film that coats all the tissues and organs and prevents or reduces the formation of surgical adhesions throughout the entire surgical area.
Data from use in surgical patients, the Phase 1 clinical trial, and preclinical studies suggest that ARC’s fluid medical devices may be used during open laparotomy, laparoscopy, and arthroscopy procedures. IPCOAT™, formerly called Discrete™, is under development for gynecological and abdominal applications. JOCOAT™, formerly called ARCJOINT™, is under development for use in orthopedic knee, shoulder, ankle and hip surgery applications. TENCOAT™ is under development for use in tendon surgeries and an additional fluid medical device is in development for spine surgeries.
Consult a health care professional
For any health condition, patients should consult their health care professional for complete information and all available treatment options, including non-medical device options.
